HISTORIC - MALARIA VACCINE ENDORSED TO PROTECT AFRICAN CHILDREN
October 10, 2021 | News release | Reading time: 2 min
The World Health Organisation (WHO) has approved the first-ever malaria vaccine that could save the lives of tens of thousands of children in Africa each year. Malaria kills about 500,000 people each year, about half of them are children in Africa. After successful pilot immunisation programmes in Ghana, Kenya and Malawi, the WHO says the vaccine should be rolled out across sub-Saharan Africa and in other regions with moderate to high malaria transmission. “This is a historic moment. The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control”, said WHO Director-General, Dr. Tedros Adhanom Ghebreyesus. “Using this vaccine on top of existing tools to prevent malaria could save tens of thousands of young lives each year”, he added. The Director of the Kintampo Health Research Centre, Dr. Kwaku Poku Asante, who has played an active role in the trials and pilot evaluation of the vaccine in Ghana said: Today is an exciting day and historic because African scientists and their partners have spent years of their lives to find a solution to malaria that has affected many lives. “It’s been a huge collaborative effort. We are grateful to all partners and collaborators in this effort especially children and their parents who participated in the trials and subsequent evaluation. We are hopeful that funding decisions for wider use will be made quickly and governments in Africa will take this recommendation as an additional tool to malaria control”, he added.
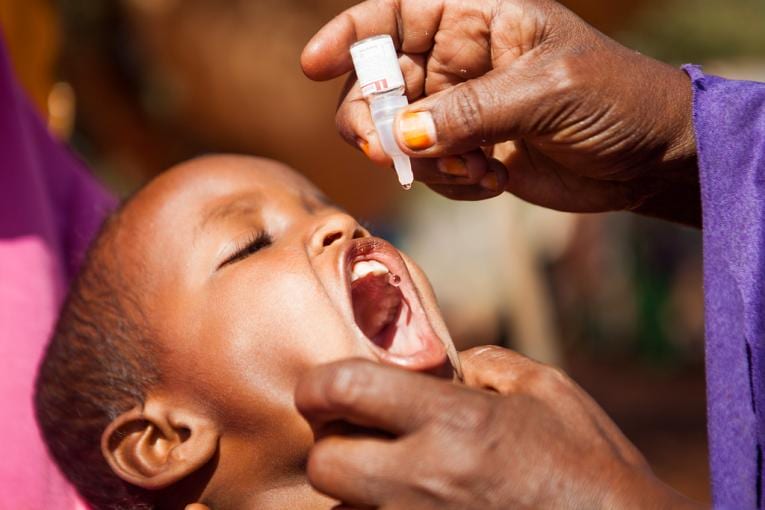
Malaria Vaccine given to children in Africa
The vaccine called RTS,S (Mosquirix) is made by GlaxoSmithKline and is given in four doses. The first three are given a month apart at five, six and seven months old, and a final booster at around 18 months. There are more than 100 types
of malaria parasite. The RTS,S acts against plasmodium falciparum, the most deadly malaria parasite globally, and the most prevalent in Africa. The next steps for the WHO-recommended malaria vaccine will include funding decisions from
the global health community for broader rollout, and country decision-making on whether to adopt the vaccine as part of national malaria control strategies. In a related development, PATH has applauded the WHO for recommending broader
use of the malaria vaccine. “It is gratifying to know that a malaria vaccine developed specifically for African children could soon be more widely available,” said Dr. Nanthalile Mugala, PATH’s Chief of the Africa Region. “This is
especially true now when progress in combating malaria has stalled in parts of the Africa region and children remain at increased risk of dying from the disease”.
